What are ferrofluids?
Ferrofluids are quite complex fluids that display interesting behavior in the presence of magnetic fields. These ferromagnetic fluids are created through the colloidal suspension of ferromagnetic particles of the nano-scale. What does this all mean? In simpler terms, you essentially take tiny (nano-scale, or on the scale of a nanometer/a billionth of a meter; think the size of the base width of a single strand of DNA) magnetic particles and disperse them homogenously (or evenly) throughout a carrier fluid in a way so that the particles are fully wetted (meaning that the particles’ surfaces are fully coated by the liquid, without other particles in dry contact with them directly).
How do ferrofluids work?
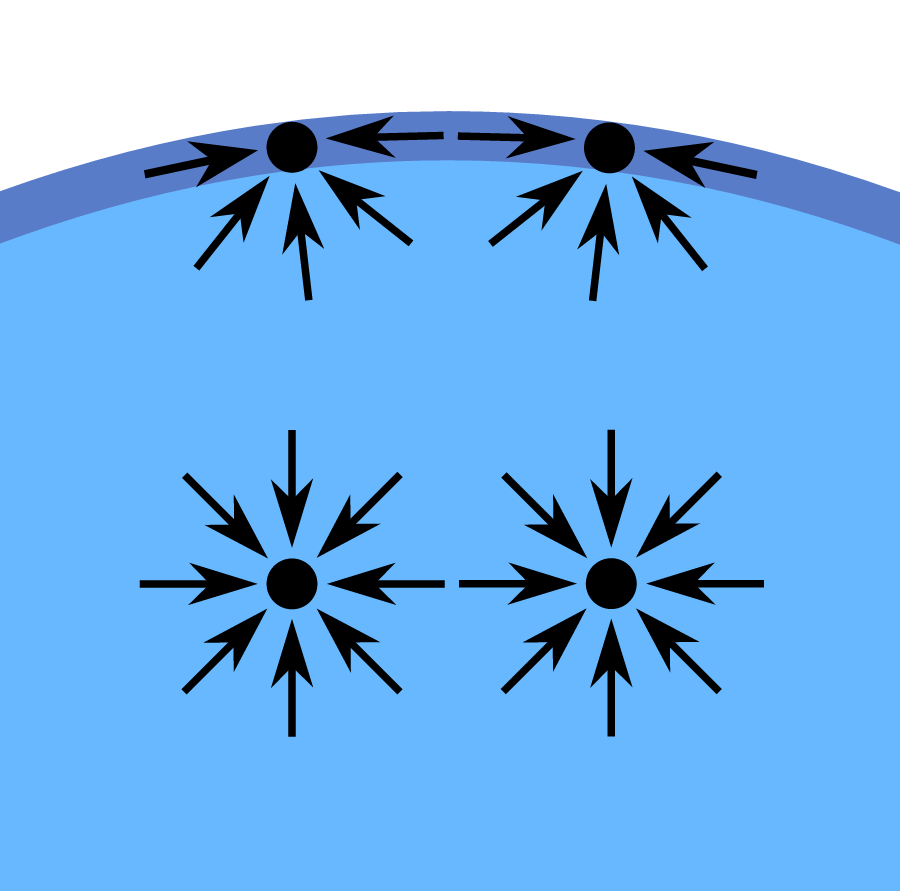
Once the ferrofluid has been created, the next step is as simple as subjecting the fluid to a magnetic field, at which point the ferrofluid becomes magnetized. As the ferrofluid begins to be affected by the magnetic field, it wants to follow this field and comply with its geometry; basically, the fluid wants to become shaped like the field it is being subjected to, but there is a problem: the fluid has surface tension. Because the fluid has cohesive bonding between liquid molecules, the molecules are very strongly attracted to each other on the molecular scale. This should make sense to you, as when you run your hand through water (for example), you are able to readily cause bulk disturbances (you can split up the water on the large scale), but try as you might, you will not be able to split the water apart on a molecular scale [the smallest you can get water to by hand is tiny visible droplets, which are still collections of a ridiculously large amount of water molecules (on the order of sextillions, or thousands of thousands of thousands of thousands of thousands of thousands of thousands of molecules in one drop!)].
Because of the aforementioned strong attraction between the liquid molecules, fully immersed liquid molecules are pulled on by other molecules in all directions, as shown in the picture above. However, the molecules on the surface are only pulled on by molecules around and below (but not above) them, leading to a breach in the equilibrium and causing the water to be pulled in the direction of the rest of the water (hence the curved water surface exhibited in the above diagram). So, armed with this knowledge of how surface tension works, we can revisit the ferrofluids and figure out what is going on.
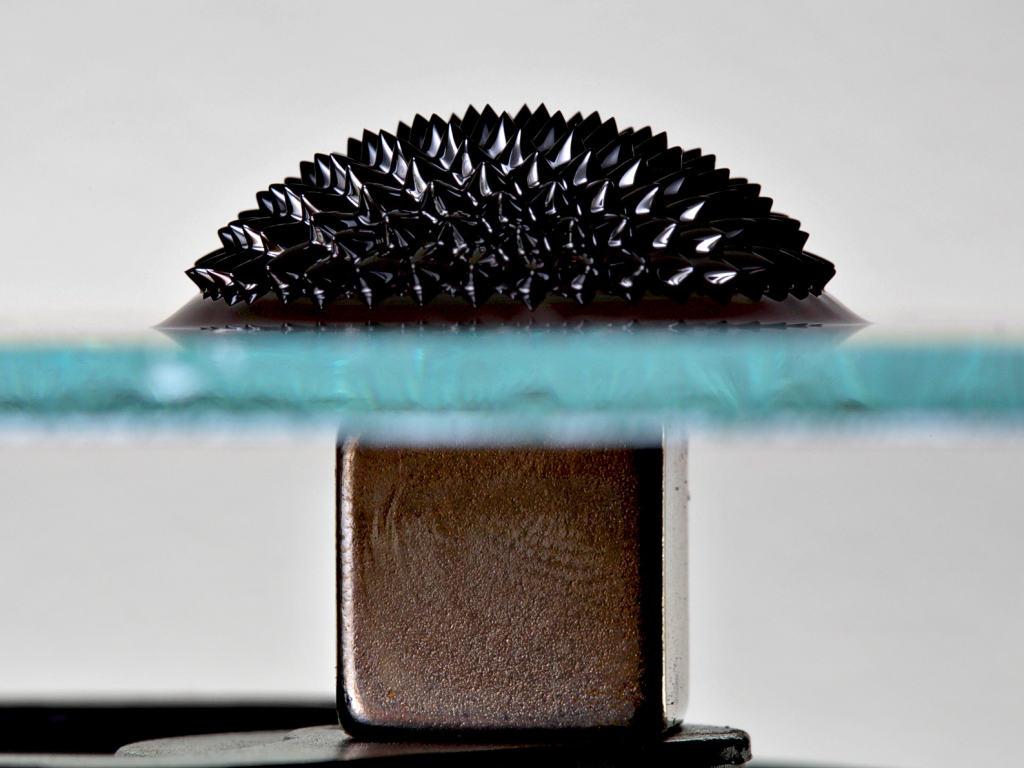
How are ferrofluids used?
The magnetic field wants to push the ferrofluid outwards, but the ferrofluid itself wants to pull itself back inwards towards the liquid base due to the surface tension, all while gravity is also resisting the spike formation (this kind of interaction is explained through the normal field instability). At some point all of these forces equate and the fluid is said to be in equilibrium. The result of this? Really cool looking spikes in the ferrofluid as shown above. This gets even cooler when sculptures are created (as in the top picture) by using shaped bases and manipulating the shape of the magnetic field. It should be noted that art is most definitely not the only application for these fluids; in fact, ferrofluids also find application as: liquid seals around the shafts of spinning hard disks/drives, as a convective heat transfer fluid for wicking away heat in small scale and low gravity applications, as an imaging agent in some medical imaging techniques (especially magnetic resonance imaging, or MRI), as friction reducing agents, as mass dampers to cancel out vibrations, and even as miniature thrusters for small (nanoscale) sattelites!
So there you have it, another awesome engineering phenomenon! Enjoy the video below, created by altering the magnetic field in a ferrofluid sculpture!